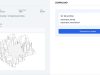
The US company Vy Spine has received approval from the US Food and Drug Administration (FDA) for its LumiVy OsteoVy PEKK Lumbar IBF spinal implant. The special feature of this implant is its material and the way it is manufactured. It is made of the high-performance plastic PEKK (poly-ether-ketone-ketone) and is manufactured using 3D printing.
The implant is used to stiffen one or two neighbouring vertebral segments in the lumbar spine area. It is used for degenerative disc disease, even in cases of first-degree spondylolisthesis. Vy Spine offers the implant in various sizes and shapes in order to be able to use it for different surgical approaches and anatomical conditions.
PEKK has several advantages over conventional PEEK. According to Vy Spine, it promotes bone growth, does not interfere with X-ray images and does not lead to the formation of connective tissue membranes. It is also said to have greater strength. Another advantage is its bacteriostatic effect, which inhibits the growth of bacteria.
The surface of the implant has a special grid structure, which Vy Spine calls ‘OsteoVy’. This is intended to additionally promote bone ingrowth. As PEKK is hydrophilic, i.e. it attracts water, the structure also supports the distribution of fluids in the implant.
Subscribe to our Newsletter
3DPresso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.